英文原文转自AACR官方博客CANCER RESEARCH Catalyst,并在 2018年1月3日发布于AACR网站,翻译仅供参考! 2017年是丰收的一年,我们在肿瘤学领域看到了许多“第一次”。其中,癌症的研究和治疗取得了诸多革命性的进步:美国食药监局第一次批准了免疫疗法,该疗法基于生物标志物来治疗癌症病人,而非过去的基于肿瘤起源部位;第一个CAR T细胞免疫疗法的批准;批准设计全面的下一代伴随诊断试验,来判断适合分子靶向疗法的病人。另一项随后被批准的试验是FoundationOne CDx试验,它可以探测任何实质瘤体中的324个基因的基因突变和两个基因签名。去年,“生物仿制药”这种癌症药物也是首次获批研发,将来这种药物很可能会降低癌症治疗的经济负担。 不过我们的未来之路还很漫长。尽管免疫疗法在某些病人体内可激发长时间的响应,仍有相当一部分病人对这些药剂并不敏感;而也有些早期有响应的病人最后产生耐药性;目前我们仍旧没有精准的生物标志物来预测产生响应的病人群体。靶向疗法也遇到同样的问题,治疗的耐药性一直以来都是比较大的难题。说了那么多,最不能忽视的是,弱势群体和缺医少药的地区,病人经常无法得到有效的癌症预防、诊断和治疗技术。 我们采访了免疫疗法和精准医疗领域的专家,癌症研究未来的发展方向是什么,2018年又会给我们带来什么重大突破,战胜癌症离我们还有多远 2018年,免疫疗法的进步 Elizabeth M. Jaffee博士 AACR候任主席Elizabeth M. Jaffee博士告诉我们:“我们已经开始基于基因学和免疫系统的理论来定义癌症”。 Jaffee博士是Dana 和 Albert “Cubby” Broccoli肿瘤学教授、约翰霍普金斯大学医学院和该大学Sidney Kimmel 综合癌症中心病理学教授、该大学癌症免疫疗法Bloomberg~Kimmel研究所副主任她预测在2018年,抗PD1/PDL1和抗CTLA4抗体疗法将获批投入应用,可单药使用亦可结合使用,用于各种癌症的小的子类型的治疗。 免疫疗法的批准速度之快前所未有,尤其是靶向PD-1/PD-L1通路的单克隆抗体。第一个抗PD1药物是在2014年获批使用的,目的是为了治疗黑色素瘤;这一类型的疗法现在已经批准用于10种类型的癌症治疗中。Jaffee 博士表示:“免疫疗法已应用到更多的癌症治疗中,从黑色素瘤或肺癌,到突变负荷较高的遗传型癌症的子类型。抗PD1/PDL1抗体在这些治疗过程中都有不错的疗效”。她预测,不仅仅是高度微卫星不稳定肿瘤(MSI-High)的治疗,在不久的未来政府相关部门将批准更多的与免疫疗法相关的生物标志物。 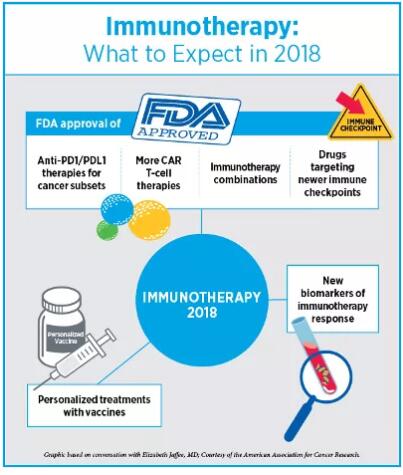 2018年,Jaffee看好的另一个值得期待的领域是更新的免疫疗法靶向。随着我们对PD1/PDL1路径更深入的掌握,研究者已经开始研究肿瘤可用的免疫响应的其他刹车系统。Jaffee告诉我们目前已有几种有效抗体处于临床试验阶段。更新的抗体包括靶向单核细胞的抗体,如CSF1R抑制剂,还有一些靶向代谢路径的抗体,如CD73抑制剂。 Jaffee 表示:“靶向新的免疫调节路径的疗法目前的研究进展良好,所以我推断,抗体应用于带有抑制单核细胞的癌症的治疗方案中,至少有一种疗法可以获得批准”。 Jaffee还表示:“今年,更多的免疫疗法的结合疗法将获得批准”,抗PD1和抗CTLA4的抗体结合疗法已被批准应用于黑色素瘤的治疗。“我认为结合疗法是多种多样的,不仅仅是这两种抗体,也有可能是其他免疫响应抑制剂。” Jaffee推测,靶向T细胞活化剂(如OX40和CD137)的药剂方面的研究,研究人员也会有比较大的进步。PD1和CTLA4会抑制T细胞的反应,而上述活化剂的分子反而加速T细胞反应。Jaffee告诉我们,靶向这些T细胞加速器的药剂目前处于临床试验阶段,今年有望迎来第一批制剂的获批。同时更多CAR T细胞疗法会被批准应用于一小部分B细胞癌症子类型病人的治疗。 Jaffee表示,我们有望在治疗性疫苗上开展更多的临床研究,包括mRNA疫苗、DNA疫苗,以及使用病人肿瘤来开发的针对性疫苗,与其他免疫药剂结合作用进行治疗。 Jaffee同样表示,在取得进步的同时我们也面临着一些挑战。一个是,有八成的病人对免疫疗法没有响应,那我们怎么去探究其免疫系统。她说:“为了了解有响应者和无响应者之间的差异,我们做了大量的临床试验”。癌症登月蓝带小组建议政府投入更多资金支持研究人员开展更多更深入的研究来探究响应者和非响应者的差异。 Jaffee表示:“我们还需要更先进的科技来协助我们理解T细胞”。我们现在知道高突变负荷肿瘤比低突变负荷肿瘤更能对免疫疗法响应。但在肿瘤细胞的茫茫抗原中,T细胞只识别特定新抗原。Jaffee的疑问是:“哪些是高质量抗原?我们是否能靶向它们?”。 Jaffee补充:“CAR T细胞对B细胞类型癌症的疗效我们还是比较看好的,但这种技术应用于实体瘤的治疗仍有一些难题待解决,研究者们正在积极寻求答案,并已获得进展”。 Jaffee表示:“现在的科学越来越发达,我们有很多好的科研工具帮助我们巩固目前已有的进展,获得更大的进步”。她认为这也是病人接受临床试验的好机会。长久以来,病人参与临床试验都是为了自己的身体,直到最近,他们的心态开始改变,他们愿意为科研知识而做出贡献;现在,有了新疗法的诞生,病人在临床试验早期就获得临床响应也不是不可能。比如,约翰霍普金斯的额两位研究人员在一向小型阶段1研究中测试到MSI-High肿瘤对抗PD-1派姆单抗有响应;但实验数据过少,制药公司无法说服FDA批准这种药物应用于人体任何部位的MSI-High肿瘤。Jaffee告诉我们:“目前这个研究从开展到获批只有三年左右时间,现在我们所取得的进步在10年前是无法想象的”。 2018年,精准医疗取得的进步 Andrea Califano博士 哥伦比亚大学医学中心化学系统生物学Andrea Califano博士,Clyde及Helen Wu 教授告诉我们:“近来,我们正在使用联系的方式---应用统计学来联系某个事件(如肿瘤突变)与具体结果。未来几年我们很有可能实现从这种纯联系的方式转变为基于模型的方式,那么我们分析多维组学数据的能力更强,从而判断癌细胞主要机械依赖性”。 Califano表示:“今年的AACR 2018年度会议上我们会展开一项重要的研讨会,探讨临床上基于模型的方式的作用”。Califano将担任研讨会主席,同时他还告诉我们,这一领域过去几年进步迅速,很有可能不久的将来我们就能获得一些新的战略来靶向癌细胞。 Califano表示近几年另一个很值得期待的方面是精准肿瘤学的综合治疗的发展。他说:“我们深深醉心于一个念头:也许有一颗神奇的子弹可以解决癌症问题”。比如通过靶向疗法或免疫疗法可能就是这颗子弹,但Califano也提示说这种想法很傻很天真。他告诉我们:“癌症是十分复杂的,所以这需要同样复杂的方案来治疗,所以我们必须研究制定综合性的定量的方法”。 Califano推测,把基于变异的靶向疗法和免疫疗法结合,再辅以其他方式,如基于RNA和基于蛋白质组的疗法,是精准医疗更“精准”的下一步逻辑发展方向,也将最终为病人带来更宝贵的生机。  Califano 表示:“现有研究领域里最夺人眼球的‘大事件’之一是单细胞技术的运用”。这些技术将彻底改变我们看待癌症的方式。单细胞序列技术能帮助我们精准聚焦肿瘤形成细胞状态和靶向疗法后产生的耐药状态。 Califano告诉我们,研究人员一直十分重视如何通过靶向癌细胞自发的免疫逃避机制来对免疫响应再激活,并积极利用基因组方法来理解这一难题。研究癌细胞自身的免疫逃避机制,其成果将大大地造福于病人,比如能减少靶向整个免疫系统的治疗方法所导至的毒性。Califano告诉我们,除此以外,一些实验室正在开发更好的生物标志物来预测免疫疗法响应,其假阳性和假阴性都在可接受范围内,临床应用前景很乐观。 Califano告诉我们,液体活检也渐渐整合为精准肿瘤学的范畴。比如,相比于传统的影像学方法,利用液体活检技术能更早地来检测到复发,那么肿瘤学医生在肿瘤变化之前就能提前决定治疗方法的调整,我们控制疾病的能力又提高一步。 关于人工智能在协助医生确定治疗方案方面,Califano还提出了一些应用挑战。大部分AI方法是用的是没有约束的基于机器学习的方法,完全是数据驱动,大大忽略了癌症生物学造成的机械约束,这是一个很大的限制。Califano补充道:“根据癌细胞潜在调控逻辑,使用生物学驱动的模式可以极大地简化我们推断现实结论的过程,这比没有约束的机器学习方法要有效很多”。Califano表示,举例来说,纵观藏匿着基因变异的基因组的所有位置,我们可以得到大量的候选者,然而,当我们结合信号和癌细胞调控逻辑来考虑其中哪些能实实在在产生相关后续影响的时候,会发现真正相关的位置只有一小撮。 Califano 表示:“使用AI,你就等于从零开始,一切皆有可能,这样你等于抛开了生物学知识,刻意忽视了癌症生物学复杂性。那么,特定的基因组背景和特定药物的敏感性之间的关联就难以被发现,就好比在大型超市里分析采购模式,一头雾水”。 Califano向我们解释:“组成超市采购模式数据的个体元素之间的联系较稀疏;然而在癌细胞生物领域,各个元素之间的联系极其复杂,每个蛋白质都和上百个其他蛋白质和代谢分子发生反应,还很有可能影响到上千个调控靶向基因。即使我们使用了超简化模式,比如一个癌细胞里的大约20,000个基因只有on和off两种状态,可即便如此,每个细胞状态也有2的20,000次幂种可能。要知道,宇宙中原子数量也不过2的250次幂。为了不在复杂的地形中‘走丢’,细胞依靠特别复杂的分子互动网络来把活性状态减少至几千种。建立复杂的没有约束的预测算法也是不可行的,且会大大限制我们的预测精准性”。 Califano告诉我们:“生物学十分复杂,这就要求需要更新颖的范例来分析和解释大的数据集,无论是从基因组角度还是从计算分析角度。让我十分振奋的是,这个领域的进步和发展方向最终可在定量和预测的基础上,实现个体化的癌症护理”。 英文原文 This past year has been a year of many “firsts” for the oncology community. The field witnessed several revolutionary advances in the research and treatment of cancer, including the approval, for the first time by the U.S. Food and Drug Administration (FDA), of an immunotherapeutic to treat patients based on biomarkers rather than the site of tumor origin; the approval of the first CAR T-cell immunotherapy; and the approval of a comprehensive next-gen companion diagnostic test to identify the right patients for the right molecularly targeted therapeutic. Another test approved later, the FoundationOne CDx test, can detect genetic mutations in 324 genes and two genomic signatures in any solid tumor type. Last year also saw the approval of first “biosimilar” cancer drugs, which could potentially lead to driving down the costs of some cancer treatments. But there is so much more to do. While immunotherapies lead to long-lasting responses for some patients, a sizable portion of patients do not respond to these agents; some patients who respond ultimately develop resistance; and we still do not have precise biomarkers to predict who will respond and who will not. The same can be said about targeted therapies, where challenges with treatment resistance continue to be a major roadblock. Above all, patients from under-represented and underserved communities quite often do not benefit from cancer prevention, diagnosis, and treatment advances. We asked experts in the fields of immunotherapy and precision medicine where the cancer research community is headed next and what major accomplishments we might expect in 2018 to take us closer to conquering cancer. Immunotherapy advances in 2018 Elizabeth M. Jaffee, MD "We are starting to define cancers based on what their genetics tells us and how the immune system sees them," says AACR President-Elect Elizabeth M. Jaffee, MD. Jaffee, who is the Dana and Albert “Cubby” Broccoli professor of oncology and professor of pathology at Johns Hopkins University School of Medicine and the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, and associate director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, predicts that this year we will see approval of anti-PD1/PDL1 and anti-CTLA4 antibody therapeutics, as single agents or as combinations, for smaller subsets of different cancers. The pace at which immunotherapeutics, particularly monoclonal antibodies that target the PD-1/PD-L1 axis, are being approved is unprecedented. The first anti-PD1 antibody was approved in 2014 to treat melanoma; this class of therapeutics has now been approved to treat 10 different types of cancers. “We are going from treating melanoma or lung cancer to treating smaller subsets of genetically linked cancers that have a high mutational burden, where anti-PD1/PDL1 antibodies work very well,” notes Jaffee. She predicts that we might see more biomarkers associated with the immunotherapeutics being approved, in addition to MSI-High tumors. 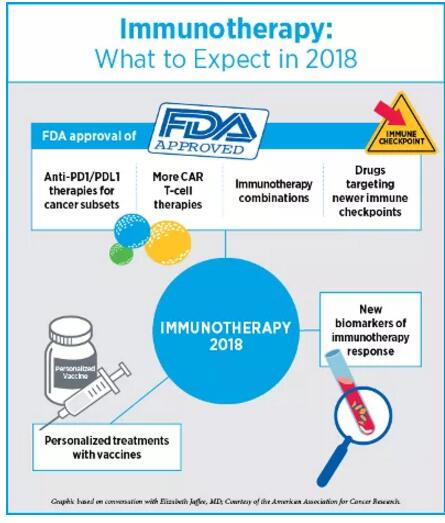 The second area to look forward to in 2018 are newer immunotherapy targets, Jaffee says. As our understanding of the PD1/PDL1 pathways continues to expand rapidly, researchers are now starting to study other brakes on the immune response that a tumor can use. There are currently several new antibodies being tested in clinical trials that are showing activity, notes Jaffee. The newer antibodies include those that target monocytes, such as the CSF1R inhibitor, and some that target metabolic pathways, such as the CD73 inhibitor. "Therapeutics targeting these new immune-modulatory pathways are looking good in early studies, so I suspect that we may see some of these antibodies approved at least in one of the many cancers that have suppressor monocytes," Jaffee says. "We are going to see more immunotherapy combinations being approved this year," adds Jaffee. A combination of anti-PD1 and anti-CTLA4 antibodies is already approved for melanoma. "I think we are going to see more of that but not just with those two types of antibodies but perhaps with other inhibitors of immune response." We are likely to make progress with other groups of agents that target activators of T cells, such as OX40 and CD137, Jaffee speculates. Unlike PD1 and CTLA4 that downregulate T cells, these molecules accelerate the T cells. Agents targeting these T-cell accelerators are being tested in clinical trials and we may see the first ones being approved this year, she notes. We might also witness more CAR T-cell therapies approved for B-cell cancers targeting small subsets of patients, she says. We are likely to see more clinical studies on therapeutic vaccines, including mRNA vaccines, DNA vaccines, and those that involve utilizing patients' tumors to develop personalized vaccines, in combination with other immune agents, notes Jaffee. There are a couple of challenges as we make progress, she said. One is understanding the tumors and immune system of the 80 percent of patients who are not responding to immunotherapies. "We are conducting many clinical studies to understand responders versus non-responders," she notes. One of the Cancer Moonshot Blue Ribbon Panel recommendations was to fund more studies that help understand the biological differences between responders and non-responders, notes Jaffee. "We also need better technologies to understand what it is that the T cells are seeing," she notes. We now know that tumors with high mutational load are likely to respond better to immunotherapies that those with a low mutational load. But the T-cells only recognize certain neoantigens among the thousands of them present on the tumor cells. "But what are these good-quality neoantigens? Can we target them?" asks Jaffee. While we are very optimistic about CAR T-cell therapies for B-cell type cancers, using this technology to treat solid tumors pose several challenges, and researchers are beginning to make progress, adds Jaffee. "There is a lot of good science coming out right now, and I think we have the right tools to follow up on these advancements to make noticeable progress,” says Jaffee. This is also a great time for patients to go on clinical trials, she explains. Until recently, patients participating in a clinical trial were not doing it for themselves but for future knowledge; now, with these new therapeutics, it is not that unlikely that patients will have a clinical response in early-stage clinical trials. As an example, the hypothesis that MSI-High tumors would respond to the anti-PD-1 pembrolizumab was tested in a small phase I study at Johns Hopkins by two investigators; but data from this small study were adequate for the pharmaceutical company to get FDA approval to treat MSI-High tumors anywhere in the body with this drug. “The total time from starting the study to approval was about three years!” exclaims Jaffee. “This is just an example of how we are really making progress today that we couldn’t have imagined 10 years ago." Precision Medicine Advances in 2018 Andrea Califano, PhD "For a while now, we have been using approaches of association – application of statistics to associate an event, such as a mutation in the tumor, with a specific outcome," begins Andrea Califano, PhD, Clyde and Helen Wu Professor of Chemical Systems Biology at Columbia University Medical Center. "One of the things that's going to happen in the next couple of years is that we are going to see a transition from such purely associative approaches to approaches that are more and more model-based, which can provide us with greater ability to analyze multi-dimensional omics data to identify the key mechanistic dependencies of cancer cells," he notes. "We are running a major symposium this year at the AACR Annual Meeting 2018 that is dedicated to addressing the role of model-based approaches in the clinics," says Califano, who is chairing the session. There have been significant scientific contributions in this area in the last few years that may lead to novel strategies for targeting cancer cells, according to him. Another thing to look for in the near future are more integrative approaches to precision oncology, according to Califano. "We have all been infatuated with the idea that there may be a single magic bullet for solving the cancer problem," for instance by using targeted therapy or immunotherapy approaches, but such views are "rather simplistic," Califano cautions. "Cancer is a complex disease requiring equally complex solutions that can only be achieved by embracing an integrative and quantitative approach." Califano speculates that integrating mutation-based targeted therapy and immunotherapy approaches with complementary approaches, such as RNA- and proteomic-based ones, is the next logical step for making precision oncology more 'precise' and ultimately more valuable to the patient." 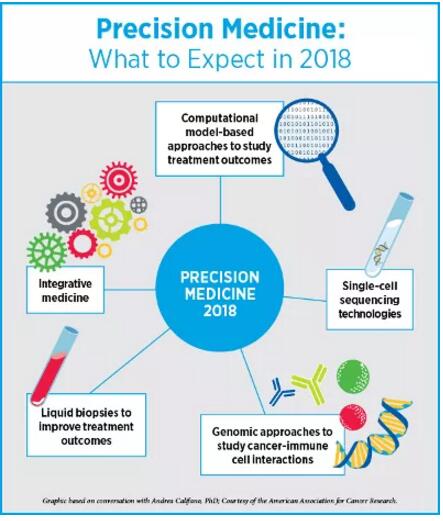 "One of the most compelling 'big things' emerging in the current research landscape is the use of single-cell technologies," notes Califano. These approaches are going to revolutionize the way we think about cancer, he says. Single-cell sequencing technologies will allow us to focus on tumor-initiating cell states and on drug-resistant states that emerge following targeted treatment, he adds. There is also significant attention on the utilization of genomic approaches to understand how to reactivate immune responses by targeting the cancer's cell autonomous contribution to immunoevasion, Califano explains. Focusing on the cancer cell-autonomous compartment that induces immune evasion will provide critical insights that could in the future benefit patients in significant ways, he says, for instance by reducing the toxicity associated with approaches that target the immune system as a whole. In addition, several laboratories are developing better biomarkers to predict immunotherapy response with acceptable ranges of false-positive and false-negatives that will support their clinical application, he notes. Finally, liquid biopsies are becoming integral to the landscape of precision oncology, Califano says. Utilizing liquid biopsies, for instance to detect relapse months earlier than currently possible with traditional methods, such as imaging, allowing the oncologist to switch treatment before the tumor has a chance to become further differentiated, represents a potentially enormous contribution to our ability to better control this disease, he says. Califano also points to the challenges with utilizing artificial intelligence (AI) in assisting physicians with identifying the right treatments for patients. Most AI approaches use unconstrained machine-learning based approaches that are completely data-driven but largely ignore the mechanistic constraints imposed by the biology of cancer, a major limitation, as he explains. "The use of biology-driven models, for instance based on the underlying regulatory logic of cancer cells, can dramatically simplify our ability to extrapolate realistic conclusions, compared to unconstrained machine learning methods" he adds. For instance, looking at all the sites in the genome that harbor a genetic alteration, can produce a huge number of candidates. However, when considering which of these can actually produce relevant downstream effect, given the signaling and regulatory logic of the cancer cell, can limit the truly relevant ones to only a handful, he notes. "By using AI you are essentially starting from a 'white board' where everything is possible, thus depriving yourself of the wisdom of biological knowledge," Califano says. "You are largely ignoring the complex biology of cancer, thus treating the problem of discovering an association between a given genomic background and sensitivity to a given drug the same way as analyzing purchasing patterns in a supermarket." The relationship between the individual elements that make up the data on supermarket purchases are relatively sparse; however, in cancer cell biology, the relationships are extraordinarily complex with each protein interacting with hundreds of other proteins, metabolites, and possibly affecting thousands of regulatory target genes, explains Califano. "Even if we used an ultra-simplified model where the roughly 20,000 genes in a cancer cell can only be in 'on' or 'off' state, each cell could exist in one of 2 to the power of 20,000 possible states. Compare that with the about 2 to the power of 250, which is the total number of atoms in the universe. To avoid being 'lost' in this extraordinary complex landscape, cells rely on an extraordinarily complex web of molecular interactions that reduce the number of viable states to only a few thousands. Building complex predictive algorithms that ignore these constraints can either be unfeasible or significantly limit our ability to be precise in our predictions," he adds. "The complexity of biology requires novel paradigms for analyzing and interpreting large data sets, both from a genomic perspective and from a computational analysis perspective, and I am very excited about the possibilities and the direction the field is taking to ultimately individualize cancer care on a truly quantitative and predictive basis," Califano says. (注:本文转自“AACR美国癌症研究学会”) |
 /3
/3 